36+ Molar Absorptivity Calculator
If the isoprene spectrum on the right was obtained from a dilute hexane solution c 4 10-5 moles per liter in a 1 cm sample cuvette a simple calculation using the above formula indicates a molar absorptivity of 20000 at the maximum absorption wavelength. Dan got 161 because he kept all the digits in his calculator and did not round off until the end.

Using The Beer Lambert Law To Calculate The Concentration Of A Solution Chemistry Study Com
3 Temperature-Concentration T-x Diagram for Zeotropic Mixture Internal energy can be calculated from u h pv.

. Absorptivity - absorption cross section of extinction coefficient which is the absorbance of a solution per unit path length and concentration. In this example the molar mass of CO 2 is about 44 gmol. Indeed the entire vertical absorbance scale may be changed to a molar absorptivity scale once this information about.
The result is the number of moles in your element or compound. The molecular mass of NH 4 2 S is 6817gmol. For example oxygen has a relative atomic mass of 159994 amu.
Find the relative atomic mass of each element in the molecule. YOU MAY USE YOUR CALCULATOR FOR THIS SECTION. K 1 008206 L atm mol 1.
Antimony is the name for the element with atomic number 36 and is represented by the symbol Kr It is a member of the metalloid group. Questions 13 are long free-response questions that require about 23 minutes each to answer and are worth 10 points each. BASICS OF HEAT TRANSFER 1 1-1 Thermodynamics and Heat Transfer 2 1-3 Heat and Other Forms of Energy 6 Energy Balance for Closed Systems Fixed Mass 12 Energy Balance for Steady-Flow Systems 12 Surface Energy Balance 13.
Divide 2 by 6817 and you have 00293 moles of NH 4 2 S. Molar absorptivity. The theoretical yield of the experiment is 367 grams of CO 2.
A calculator with an advanced screen. Online and blended learning opportunities in Chemical Engineering curriculum emerged due to COVID-19. S JmolK H.
The molar absorptivity of a compound at 500 nm wavelength is 252 M-1cm-1. Put on a pair of heavy gloves use a dust pan and dust broom to pick up the broken glass and put it in a broken glassware receptacle. YOU MAY USE YOUR CALCULATOR FOR THIS SECTION.
Online calculator figures and tables showing specific heat C P and C V of gasous and liquid ammonia at temperatures ranging from -73 to 425C -100 to 800F at pressure ranging from 1 to 100 bara 145 - 1450 psia - SI and Imperial Units. Pdf from CHEM 110 at Columbia College. NaOH solution is available to use as the titrant.
Take the products you obtained in the previous step and add them all together to calculate the molar mass of the compound. For carbon dioxide CO 2 the relative atomic mass is 12011 amu for carbon and 15999 for oxygen. Questions 13 are long free-response questions that require about 23 minutes each to.
The student uses a. Absorptivity - absorption cross section of extinction coefficient which is the absorbance of a solution per unit path length and concentration. Concentration of the hydrochloric acid solution must be less than the molar concentration of the propanoic acid solution.
Questions 47 are short free-response questions that require about 9 minutes each to answer and are worth 4 points each. Enter the email address you signed up with and well email you a reset link. The molar absorption coefficient ϵ molar refers to the absorbance of light per unit path length and per unit of concentration expressed in moles per liter For proteins an absorbance maximum near 280 nm A 280 in the UV spectra of a protein solution is mostly due to the presence of aromatic tryptophan and tyrosine residues.
Antimony is the name for the element with atomic number 36 and is represented by the symbol Kr It is a member of the metalloid group. K 1 6236 L torr mol 1. When entropy or enthalpy are known at a reference temperature T0 and pressure p0 values at any temperature and pressure may be obtained by combining Equations 33 and 35 or Equations 34 and 36.
YOU MAY USE YOUR CALCULATOR FOR THIS SECTION. Carbons molar mass is 12 gmol and oxygens is 16 gmol so the total is 12 16 16 44 Multiply 0834 moles CO 2 x 44 gmol CO 2 367 grams. Questions 13 are long free-response questions that require about 23 minutes each to answer and are worth 10 points each.
Questions 47 are short free-response questions that require about 9 minutes each to answer and are worth 4 points each. YOU MAY USE YOUR CALCULATOR FOR THIS SECTION. E The equipment shown above is provided so that the student can determine the value of the molar heat of solution for urea.
Knowing that the specific heat of the solution is 418 JgC list the specific measurements that are required to be made during the experiment. B path length. Molar absorption coefficient absorption cross section etc.
8314 J mol. Using a calculator divide the number of grams by the molar mass. A student is given the task of determining the concentration of a propanoic acid solution of unknown.
Questions 13 are long free-response questions that require about 23 minutes each to answer and are worth 10 points each. Enter the email address you signed up with and well email you a reset link. The molecular mass of HCl is 3646 gmol so the molarity is Molarity If you divide 1010063 0 you get 160.
On the one hand and the quantities in Maxwells equations like the dielectric function. 3646 grams is the mass of one mole of hydrogen chloride. Use a copy of the Periodic Table of ElementsThe Periodic Table lists the atomic mass of each element below the chemical symbol.
For hydrogen chloride the molar mass is 1007 35453 36460 gmol. The number 101 was really 1014 8 and 1014 80063 0 ⴝ 161. Questions 47 are short free-response questions that require about 9 minutes each to answer and are worth 4 points each.
For example imagine you have 2 g of NH 4 2 S and you want to convert it to moles. A calculator with an advanced screen. This determines the molar mass for the entire compound.
Ammonia - Thermal Conductivity vs.

Pdf Revised Uv Extinction Coefficients For Nucleoside 5 Monophosphates And Unpaired Dna And Rna
Beer Lambert Law

Transmittance Formula Transmittance To Absorbance Calculation Electrical4u

How To Calculate Molar Extinction Coefficient In Origin Youtube

How To Calculate Molar Absorptivity 8 Steps With Pictures
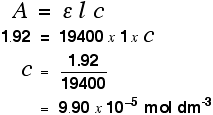
Using Uv Visible Absorption Spectra

How To Calculate Molar Absorptivity 8 Steps With Pictures

How To Find Molar Absorptivity Using The Beer Lambert Law Chemistry Study Com
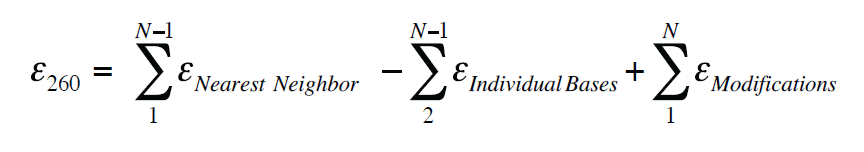
Oligo Quantification Getting It Right Idt
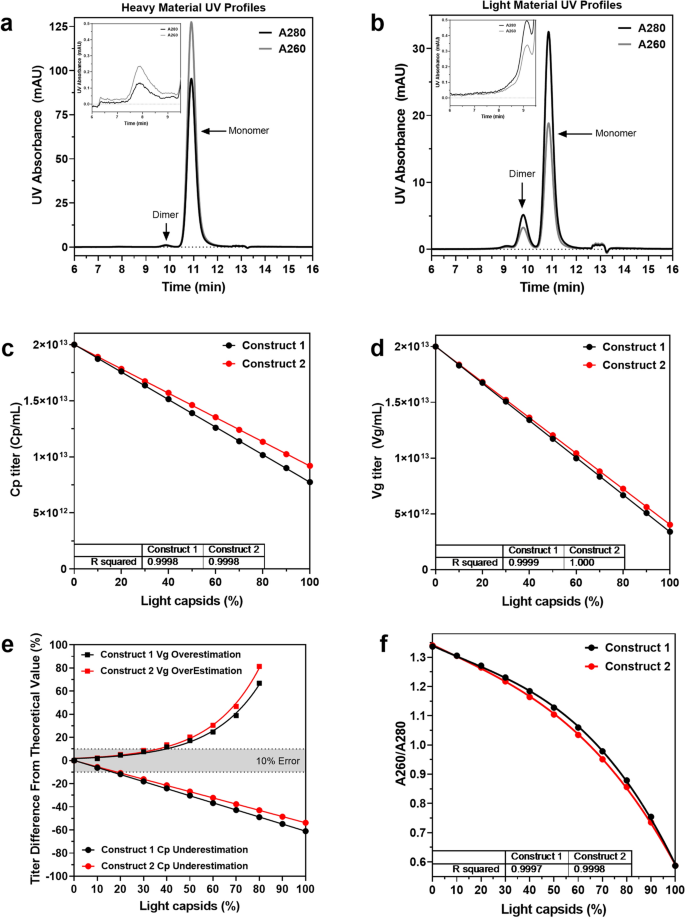
Comprehensive Characterization And Quantification Of Adeno Associated Vectors By Size Exclusion Chromatography And Multi Angle Light Scattering Scientific Reports

Absorption Coefficient A Calculation From Uv Vis Absorbance Data In Origin Youtube

Circular Dichroism Spectroscopy Units

How To Calculate The Coefficient Of Molar Absorption Sciencing

Determine Extinction Coefficient For Accurate Results

Beers Law Youtube
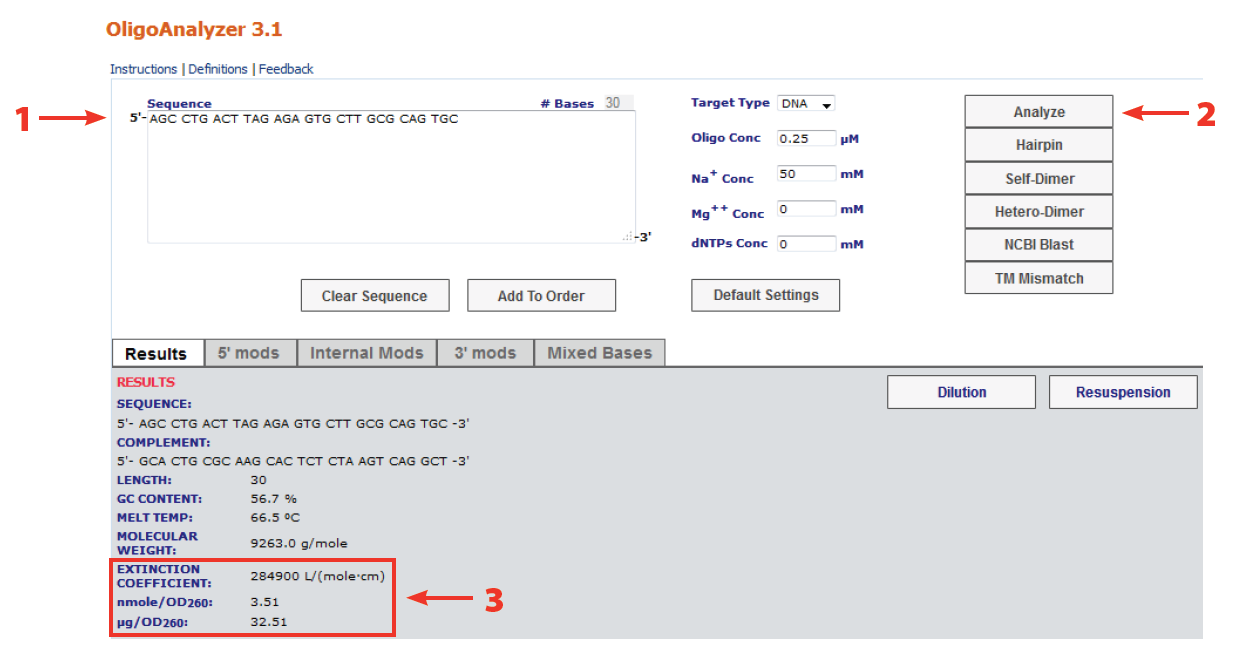
Oligo Quantification Getting It Right Idt

Calculation Of Extinction Coefficient From Absorbance Youtube